Single-cell transcriptomics of the ventral posterolateral nucleus-enriched thalamic regions from HSV-1-infected mice reveal a novel microglia/microglia-like transcriptional response | Journal of Neuroinflammation | Full Text
Intranasally administered HSV-1 H25 strain is mostly found in the thalamus and hindbrain on day 6 post-infection
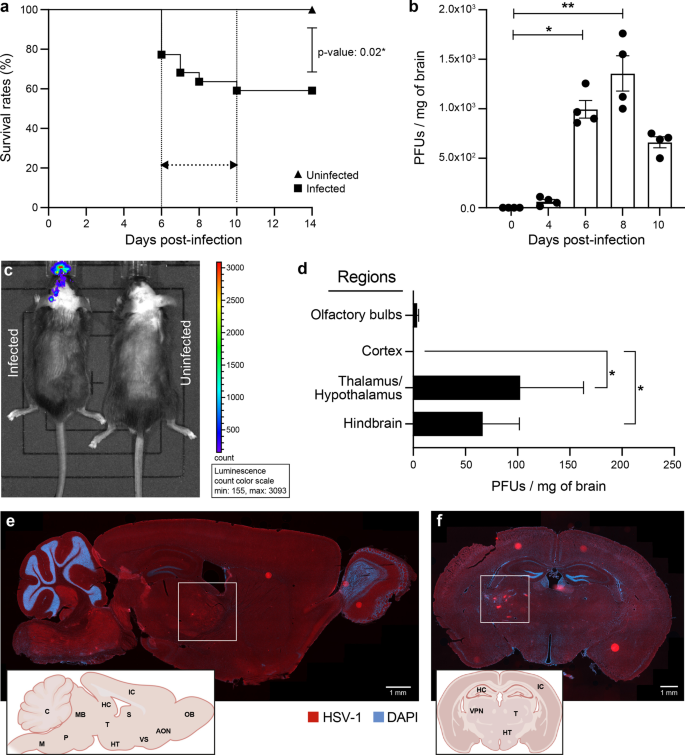
We inoculated 6-week-old C57BL/6N mice intranasally with 6 × 105 PFUs of HSV-1 H25 clinical strain. Infected mice developed a swollen forehead as their first clinical sign of the HSE on day 5 p.i. Mortality started to occur on day 6 p.i. (22.8%), then culminated on day 10 p.i. (41%). The overall mortality rate was significantly higher in the infected group (59%) than in uninfected mice (0%; P = 0.02) (Fig. 1a). On day 4 p.i., viral titers in whole brain homogenates became detectable (64 PFUs/mg of the brain). On day 6 (995 PFUs/mg of the brain), they significantly increased and reached the highest levels on day 8 p.i. (1357.5 PFUs/mg of the brain), then started to decrease on day 10 p.i. (662 PFUs/mg of the brain) (Fig. 1b).
HSV-1 replication in thalamus and hindbrain leads to increased mortality on day 6 p.i. 6-week-old C57BL/6N male mice (n = 12 mice) were intranasally infected with 6 × 105 PFUs of HSV-1 strain H25 in 20 μl MEM. a Survival curves of HSV-1-infected vs. uninfected control mice. Survival rates were analyzed using a log-rank (Mantel–Cox) test. b Viral titers in homogenates of brains were measured by a standard plaque assay on Vero cells on days 0, 4 and 6 p.i.. The results are reported as PFUs per milligram (mg) of brain homogenates and represent the means ± SEM for 4 mice per group at each timepoint. c Representative bioluminescence image of mouse infected with 6 × 105 PFUs of recombinant HSV-1 (rHSV-1) on the left and uninfected control mouse on the right side, on day 6 p.i. The bioluminescent signal is expressed in average radiance (p/s/cm2/sr). d Identification of HSV-1+ brain regions on day 6 p.i. Viral titers in homogenates of different brain regions were measured by a standard plaque assay on Vero cells. Representative sagittal (e) and coronal (f) brain sections illustrating the localization of HSV-1 proteins (red) on day 6 p.i. Brain sections were immunostained with a primary polyclonal rabbit anti-HSV-1/2 antibody and a secondary Alexa-594 conjugated anti-rabbit antibody, followed by staining with DAPI (blue) (scale bar = 1 mm). HSV-1 signal was localized in the ventral posterolateral nucleus (VPL) of the thalamus (white squares) and hindbrain (cerebellum, medulla, and pons). Brain regions are indicated on the representative sagittal and coronal mouse brain sections inserted in the left corners (AON.: Anterior olfactory nucleus, C.: Cerebellum, HC.: Hippocampus, HT.: Hypothalamus, IC.: Isocortex, M: Medulla, MB.: Midbrain, OB.: Olfactory bulb, P.: Pons, S: Septum, T.: Thalamus, VPN: Ventral posterior nucleus, VS.: Ventral striatum). f All statistical analyses were performed using Kruskal–Wallis with “Dunn’s multiple comparisons test.” Statistically significant results are indicated as follows: *P < 0.05; **P < 0.01
HSV-1 replication in thalamus and hindbrain leads to increased mortality on day 6 p.i. 6-week-old C57BL/6N male mice (n = 12 mice) were intranasally infected with 6 × 105 PFUs of HSV-1 strain H25 in 20 μl MEM. a Survival curves of HSV-1-infected vs. uninfected control mice. Survival rates were analyzed using a log-rank (Mantel–Cox) test. b Viral titers in homogenates of brains were measured by a standard plaque assay on Vero cells on days 0, 4 and 6 p.i.. The results are reported as PFUs per milligram (mg) of brain homogenates and represent the means ± SEM for 4 mice per group at each timepoint. c Representative bioluminescence image of mouse infected with 6 × 105 PFUs of recombinant HSV-1 (rHSV-1) on the left and uninfected control mouse on the right side, on day 6 p.i. The bioluminescent signal is expressed in average radiance (p/s/cm2/sr). d Identification of HSV-1+ brain regions on day 6 p.i. Viral titers in homogenates of different brain regions were measured by a standard plaque assay on Vero cells. Representative sagittal (e) and coronal (f) brain sections illustrating the localization of HSV-1 proteins (red) on day 6 p.i. Brain sections were immunostained with a primary polyclonal rabbit anti-HSV-1/2 antibody and a secondary Alexa-594 conjugated anti-rabbit antibody, followed by staining with DAPI (blue) (scale bar = 1 mm). HSV-1 signal was localized in the ventral posterolateral nucleus (VPL) of the thalamus (white squares) and hindbrain (cerebellum, medulla, and pons). Brain regions are indicated on the representative sagittal and coronal mouse brain sections inserted in the left corners (AON.: Anterior olfactory nucleus, C.: Cerebellum, HC.: Hippocampus, HT.: Hypothalamus, IC.: Isocortex, M: Medulla, MB.: Midbrain, OB.: Olfactory bulb, P.: Pons, S: Septum, T.: Thalamus, VPN: Ventral posterior nucleus, VS.: Ventral striatum). f All statistical analyses were performed using Kruskal–Wallis with “Dunn’s multiple comparisons test.” Statistically significant results are indicated as follows: *P < 0.05; **P < 0.01
To determine whether HSV-1 invades the CNS following intranasal infection, we imaged C57BL/6N mice on day 6 after intranasal infection with 6 × 105 PFUs of rHSV-1 expressing Gaussia Luciferase using an intravital imaging system (IVIS) [29]. We first noted an ongoing HSV-1 infection in the nasal cavity. Widespread viral dissemination, mainly on the left side of the brain, was observed. The caudal forebrain, midbrain, and hindbrain were the three main regions emitting significant levels of bioluminescence (Fig. 1c). Interestingly, the hindbrain signal was more intense than the olfactory bulbs, considered the first region to get infected by HSV-1 in our HSE mouse model. This observation suggests that the thalamic infection occurred earlier than in the other areas, or the immune response in the olfactory bulbs was more effective at controlling the viral infection.
To provide further insights into the HSV-1 dissemination in the CNS, viral titers were next measured in four specific regions of the brain. We found that viral replication occurred mainly in the thalamic–hypothalamic regions (102.3 PFUs/mg of the brain) and hindbrain (66.3 PFUs/mg of the brain) on day 6 p.i. (Fig. 1d). Furthermore, we aimed to determine the exact location of HSV-1-infected CNS cells. To do so, we performed IF analyses on coronal and sagittal brain sections obtained from HSV-1-infected mice on day 6 p.i. In concordance with viral titers, the thalamus, hypothalamus, cerebellum, pons, and medulla were highly stained for HSV-1 (Fig. 1e). Coronal sections from the same group of infected mice confirmed that HSV-1+ CNS cells were mainly localized in the thalamus, specifically the VPL, a subregion of VPN (Fig. 1f). Our results suggest that distinct CNS regions display a differential vulnerability to HSV-1 infection. Based on these findings, we decided to study the highly infected thalamus, especially the VPL, on day 6 p.i.
Reactive microglia are involved in the phagocytosis of HSV-1-infected cells and antigen presentation in moderately infected thalamic regions
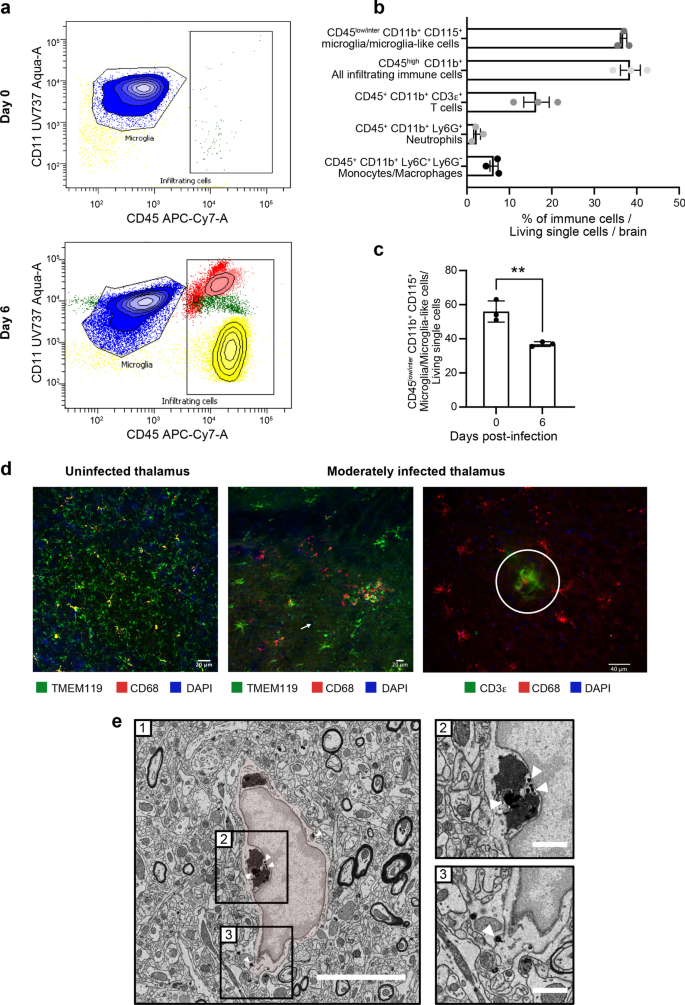
To determine the CNS composition of immune cells on days 0 and 6 p.i., we analyzed HSV-1-infected whole brains by flow cytometry. As expected, the only immune cells observed before infection were CD45intCD11b+CD115+ microglia (Fig. 2a, top). On day 6 p.i., however, we observed three other types of leukocytes, i.e., CD45+CD11b+Ly6C+Ly6G− monocytes/macrophages, CD45+CD11b+CD3ε+ T cells and CD45+CD11b+Ly6G+ neutrophils (Fig. 2a, bottom; Fig. 2b). Interestingly, the number of microglia-like cells was significantly reduced (Fig. 2c, P = 0.006), suggesting a possible elimination or phenotypic transformation of reactive microglia during HSE. We conclude that infiltrating immune cells and microglia/microglia-like cells are jointly involved in HSE control.
Reactive microglia are involved in the HSV-1+ cell clearance and antigen presentation in moderately infected thalamus. a Representative flow cytometry plots for CD45+CD11b+ gate showing infiltrating immune cell populations (T cells represented in yellow, neutrophils in red, monocytes/macrophages in light pink) and microglia/microglial-like cell (in blue) in whole brain homogenates on day 0 (top) and day 6 p.i. (bottom). b Percentages of different immune cell populations, compared to all living single cells obtained from a whole brain on day 6 p.i. c Mice infected with HSV-1 showed reduced percentages of CD45+CD11b+CD115+ microglia/microglial-like cells in the thalamus on day 6 p.i. The results represent the means ± SEM for 3 mice per group at each timepoint. Statistical analyses were performed using an unpaired t test. Statistically significant results are indicated as follows: **P < 0.001. d, left) Immunofluorescence image of uninfected VPL (day 0) immunostained for TMEM119+ (green) CD68+ (red) shows surveillant microglia. d, middle) Immunofluorescence analyses of brain sections of moderately infected VPLs on day 6 p.i. show TMEM119+ (green) CD68+ (red) microglia/microglial-like cells gathering around HSV-1+ cells (pink). TMEM119+ ameboid microglia/microglial-like cells (white arrow) are visualized. d, right) Fluorescent microscopy image shows colocalized signals of TMEM119+ (red) microglia/microglial-like cells establishing a direct contact with CD3e+ (green) T cells (white circle). Nuclear staining was done with DAPI for both images. e HSV-1+ nanoparticles are specifically found within the endosome and at the membrane of microglia/microglia-like cells in SEM images of VPL on day 6 p.i. On the representative picture of HSV1-immunopositive microglia/microglial-like cells (e-1), the microglia/microglial-like cell are pseudocolored in red, and square boxes identify subcellular compartments. (e-2, 3). Insert at higher magnification shows nanogold particles within the (e-2) endosomes containing digested contains and (e-3) at the cellular membrane. Scale bars are equivalent to 5 μm at low magnification (e-1) and 1 μm at higher magnification (e-2, 3)
Reactive microglia are involved in the HSV-1+ cell clearance and antigen presentation in moderately infected thalamus. a Representative flow cytometry plots for CD45+CD11b+ gate showing infiltrating immune cell populations (T cells represented in yellow, neutrophils in red, monocytes/macrophages in light pink) and microglia/microglial-like cell (in blue) in whole brain homogenates on day 0 (top) and day 6 p.i. (bottom). b Percentages of different immune cell populations, compared to all living single cells obtained from a whole brain on day 6 p.i. c Mice infected with HSV-1 showed reduced percentages of CD45+CD11b+CD115+ microglia/microglial-like cells in the thalamus on day 6 p.i. The results represent the means ± SEM for 3 mice per group at each timepoint. Statistical analyses were performed using an unpaired t test. Statistically significant results are indicated as follows: **P < 0.001. d, left) Immunofluorescence image of uninfected VPL (day 0) immunostained for TMEM119+ (green) CD68+ (red) shows surveillant microglia. d, middle) Immunofluorescence analyses of brain sections of moderately infected VPLs on day 6 p.i. show TMEM119+ (green) CD68+ (red) microglia/microglial-like cells gathering around HSV-1+ cells (pink). TMEM119+ ameboid microglia/microglial-like cells (white arrow) are visualized. d, right) Fluorescent microscopy image shows colocalized signals of TMEM119+ (red) microglia/microglial-like cells establishing a direct contact with CD3e+ (green) T cells (white circle). Nuclear staining was done with DAPI for both images. e HSV-1+ nanoparticles are specifically found within the endosome and at the membrane of microglia/microglia-like cells in SEM images of VPL on day 6 p.i. On the representative picture of HSV1-immunopositive microglia/microglial-like cells (e-1), the microglia/microglial-like cell are pseudocolored in red, and square boxes identify subcellular compartments. (e-2, 3). Insert at higher magnification shows nanogold particles within the (e-2) endosomes containing digested contains and (e-3) at the cellular membrane. Scale bars are equivalent to 5 μm at low magnification (e-1) and 1 μm at higher magnification (e-2, 3)
IF studies were subsequently performed in the infected VPN and its subregion VPL of the thalamus on day 6 p.i.. We focused on this thalamic region connecting the trigeminal nerve via the trigeminothalamic tract, which HSV-1 potentially uses to infiltrate the CNS [46]. The microglia/microglia-like cell populations were labeled using TMEM119, a well-known microglia marker [47]. Qualitative analysis of confocal images of moderately infected thalamus showed that TMEM119+ cells underwent morphological alterations, such as swollen cell bodies and thicker cytoplasmic processes, compared to surveillant microglia prior to the infection (Fig. 2d, left and middle). Besides these hypertrophic/reactive microglia, we also observed important numbers of TMEM119+ ameboid microglia/microglia-like cells, corresponding to a hyperreactive microglial phenotype (Fig. 2d, middle). Microglia/microglia-like cells migrated towards HSV-1+ cells and were closer to one another in the moderately infected thalamus on day 6 p.i. The increased clustering of microglia/microglia-like cells around infected cells was associated with a decreased microglial density in the surrounding parenchyma (Fig. 2d, middle and right). We also found TMEM119+ microglia/microglia-like cells expressing the phagolysosomal activity marker CD68 in moderately infected VPLs, suggesting microglial involvement in viral clearance by phagocytosis of HSV-1+ cells. Of note, most CD68+ spots were not colocalized within TMEM119+ cells, indicating the presence of other phagocytic cells with even higher lysosomal activity in the region (Fig. 2d, middle). Some of the hypertrophic/reactive microglia/microglia-like cells closely localized with CD3ε+ T cells (Fig. 2d, right), suggesting potential antigen uptake and processing for microglial antigen presentation.
To provide additional functional insights into microglial involvement in HSV-1-infected VPL on day 6 p.i., we next performed ultrastructural analysis of microglia using nanoscale-resolution SEM. Microglia were identified based on their well-established distinctive ultrastructural characteristics [48]. As this method cannot fully differentiate between microglia and infiltrating monocytes, we refer to the examined cells as microglia/microglia-like cells, as described elsewhere [21]. Nanogold staining against HSV-1 (Fig. 2e-1) revealed that the virus was mostly located within endosomes containing digested contents (Fig. 2e-2) and at the cellular membrane of microglia/microglia-like cells (Fig. 2e-3), instead of being detected in the nucleus, where the majority of HSV-1 replication takes place [49]. This result supports the hypothesis that microglia/microglia-like cells phagocytose HSV-1+ cells to limit viral replication.
Microglial functions are impaired in highly infected thalamic regions
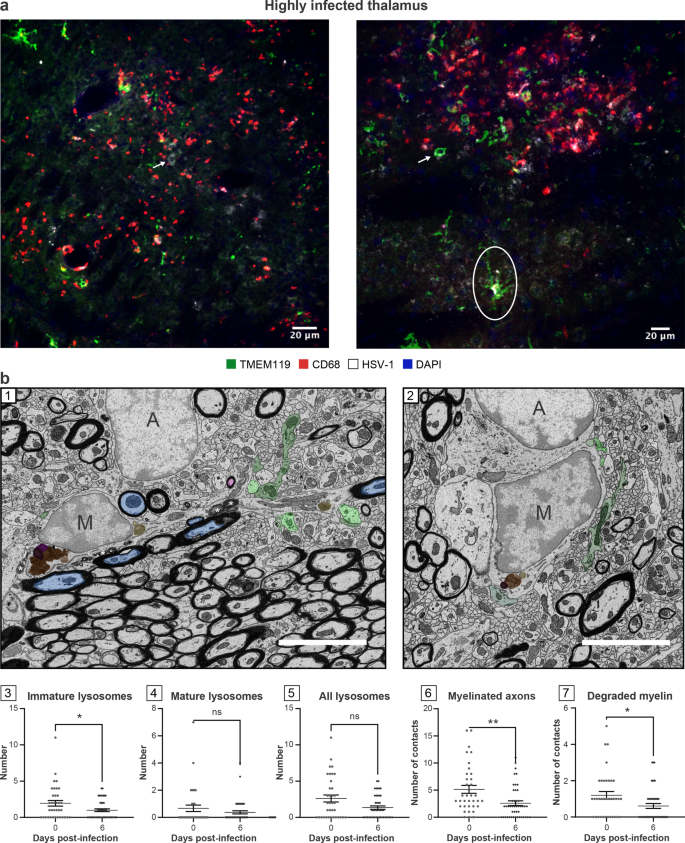
We noticed that TMEM119 staining remained present in these regions despite the inflammatory environment [50]. These areas were populated by a mixture of hypertrophic and round-shaped microglia/microglia-like cells associated with a reactive state (Fig. 3a). Qualitative analysis of highly infected thalamus on day 6 p.i. showed a reduced hypertrophic/reactive microglial density. Most TMEM119+ ameboid microglia/microglia-like cells contacting the infected cells did not express CD68 (Figs. 2d, 3a). Despite decreased numbers of TMEM119+/CD68+ microglia/microglia-like cells, the highly infected thalamus was enriched in CD68+ puncta, suggesting that unknown microglial subsets downregulating TMEM119, or other phagocytes compensate for the impaired phagocytic activity of TMEM119+ microglia/microglia-like cells (Fig. 3a) [51, 52]. This CD68 expression was positively correlated with the intensity of the infection. Moreover, detecting HSV-1 signals on TMEM119+/CD68− ramified microglia/microglia-like cells raised the intriguing possibility that these cells could be infected (Fig. 3a, right). Our results highlight an exacerbated expression of CD68, mainly found on other cells than TMEM119+ microglia/microglia-like cells in the highly infected thalamus.
TMEM119+ microglia/microglial-like cells exhibit impaired functions, including reduced phagocytic activity in highly infected thalamus. a HSV-1-infected thalamus was labeled with antibodies against TMEM-119 (green), CD68 (red), HSV-1 (white), and counterstained with DAPI (blue). (left) Highly infected thalamus immunostained with TMEM119 (green), CD68 (red), HSV-1 (white) antibodies, and counterstained with DAPI showed increased numbers of TMEM119−/CD68+ cells in close/direct contact with HSV-1+ CNS cells (white arrow) on day 6 p.i.. (right) Confocal microscopy image shows TMEM119+ ameboid microglia/microglia-like cells (white arrow) with the lack of CD68 expression. HSV-1 signal was colocalized with TMEM119+/CD68− ramified (white circle) and ameboid microglia/microglia-like cells (white arrow), suggesting an impaired phagocytic activity following microglial infection by HSV-1 or the phagocytosis of HSV-1+ CNS cells. b (1, 2) Representative pictures at day (1) 0 and (2) 6 p.i. showed that on day 6 p.i. microglia/microglial-like cells have a lower number of (3) immature lysosomes without major changes in (4) mature and (5) total lysosomes number and made fewer contacts with (6) myelinated axons and (7) degraded myelin. (1, 2) Microglia/microglial-like cell organelles and elements of their microenvironment that are in contact with the cell body are pseudocolored; immature lysosomes are in yellow, mature lysosomes are in orange, lipofuscins are in pink, lipid bodies are in dark purple, myelinated axons are in blue, presynaptic axon terminals are in light green, and postsynaptic dendrites or dendritic spines are in dark green. Myelin degradation is distinguished by a white asterisk (*). Apparent cell bodies are identified by a capital letter, where “M” stands for microglia/microglial-like cell, and “A” stands for astrocytes. Scale bars are equivalent to 5 µm. (3–7) Graphs show means (thin wide bar) ± SEM, where individual values are represented by dark gray circles at day 0 (uninfected control) and black diamonds at day 6 p.i.
TMEM119+ microglia/microglial-like cells exhibit impaired functions, including reduced phagocytic activity in highly infected thalamus. a HSV-1-infected thalamus was labeled with antibodies against TMEM-119 (green), CD68 (red), HSV-1 (white), and counterstained with DAPI (blue). (left) Highly infected thalamus immunostained with TMEM119 (green), CD68 (red), HSV-1 (white) antibodies, and counterstained with DAPI showed increased numbers of TMEM119−/CD68+ cells in close/direct contact with HSV-1+ CNS cells (white arrow) on day 6 p.i.. (right) Confocal microscopy image shows TMEM119+ ameboid microglia/microglia-like cells (white arrow) with the lack of CD68 expression. HSV-1 signal was colocalized with TMEM119+/CD68− ramified (white circle) and ameboid microglia/microglia-like cells (white arrow), suggesting an impaired phagocytic activity following microglial infection by HSV-1 or the phagocytosis of HSV-1+ CNS cells. b (1, 2) Representative pictures at day (1) 0 and (2) 6 p.i. showed that on day 6 p.i. microglia/microglial-like cells have a lower number of (3) immature lysosomes without major changes in (4) mature and (5) total lysosomes number and made fewer contacts with (6) myelinated axons and (7) degraded myelin. (1, 2) Microglia/microglial-like cell organelles and elements of their microenvironment that are in contact with the cell body are pseudocolored; immature lysosomes are in yellow, mature lysosomes are in orange, lipofuscins are in pink, lipid bodies are in dark purple, myelinated axons are in blue, presynaptic axon terminals are in light green, and postsynaptic dendrites or dendritic spines are in dark green. Myelin degradation is distinguished by a white asterisk (*). Apparent cell bodies are identified by a capital letter, where “M” stands for microglia/microglial-like cell, and “A” stands for astrocytes. Scale bars are equivalent to 5 µm. (3–7) Graphs show means (thin wide bar) ± SEM, where individual values are represented by dark gray circles at day 0 (uninfected control) and black diamonds at day 6 p.i.
In parallel, ultrastructural analysis of microglia/microglia-like cells identified based on their ultrastructure (Fig. 3b-1, 2) was conducted by quantifying the number of organelles involved in phagolysosomal pathways on SEM images. This analysis revealed that microglia/microglia-like cells possessed a significantly reduced pool of immature lysosomes (also known as early endosomes) in the VPL on day 6 p.i. compared to day 0 (Fig. 3b-3, P < 0.05), whereas no significant change was seen for other organelles (Fig. 3b-4, 5; Additional file 1: Fig. S1, organelles). This observation supports the HSV-1-mediated disruption of microglial phagolysosomal pathways during HSE. Next, we evaluated the interactions that microglia/microglia-like cells established with their microenvironment, including CNS cells, such as other microglia/microglia-like cells, neurons, astrocytes, but also their subcellular compartments like myelinated axons, on the same SEM images (Additional file 1: Fig. S1, contacts). A reduced number of microglia/microglia-like cells made direct contact with myelinated axons (Fig. 3b-6, P < 0.01), but also with degraded myelin (Fig. 3b-7, P < 0.05) in the infected VPL. Taken together, we hypothesize that critical functions of microglia/microglia-like cells, such as the monitoring of neurons and clearance of myelin debris through phagocytosis, were impaired by a highly inflammatory environment in the HSV-1-infected thalamus.
Identification of a novel microglia-like transcriptional signature in HSE
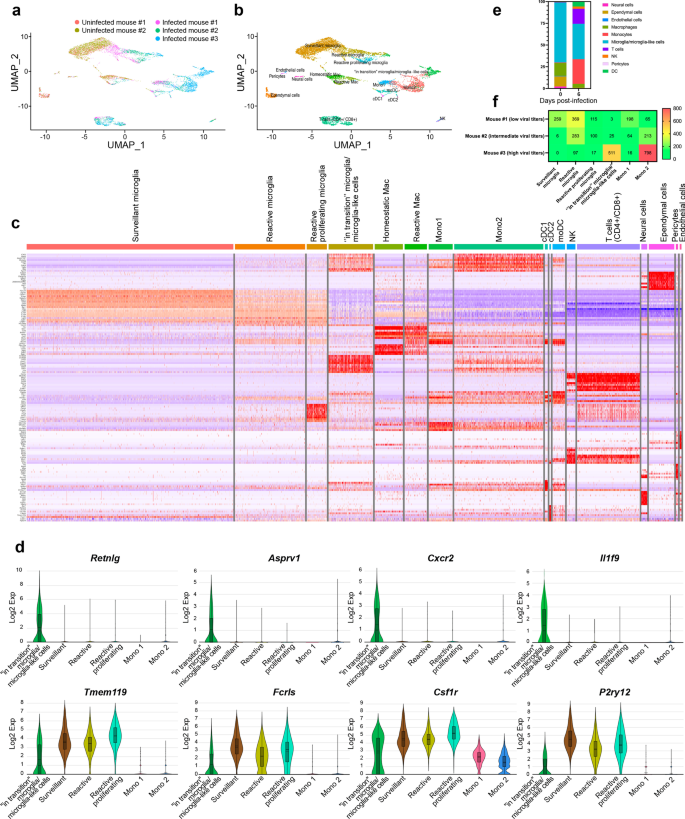
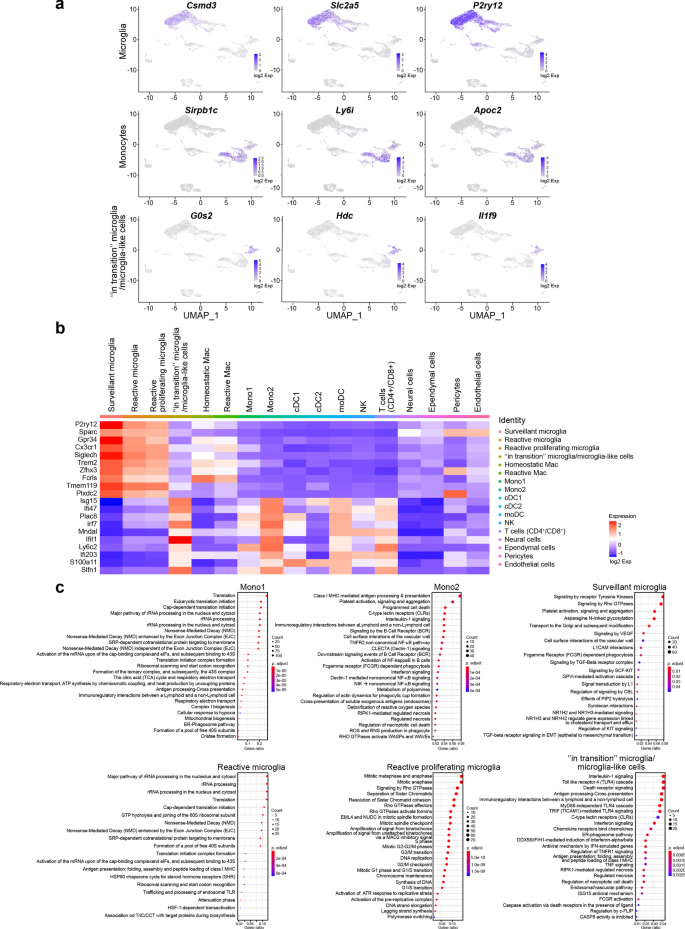
Our scRNA-seq analysis on CD11b+ cells isolated from the thalamus on day 6 p.i. provides a broad view of the transcriptional response of CD11b+ immune cells involved in the immune response during HSE. We mapped 7658 cells of an aggregated scRNA-seq data set consisting of five samples. Ten cell populations were identified based on UMAP and cell-specific markers (Additional file 2: Fig. S2a), i.e., T cells (Cd3d, Trbc2, Trac), natural killer cells (NK) (Klra8, Klra4, Klrb1c), monocytes (Sirpb1c, Ly6i, Apoc2), dendritic cells (DCs) (Kmo, H2-DMb2, Ccr7), macrophages (mac) (Pf4, Mrc1, Cd163), microglia (P2ry12, Slc2a5, Sall3), pericytes (Vtn, Ndufa4l2, Pdgfrb), ependymal cells (Ttr, Enpp2, Ecrg4), endothelial cells (Ptprb, Ly6c1, Cldn5) and neural cells (Ptprz1, Gpr37l1, Ntsr2).
Individually analyzed samples revealed that for each sample, major cell clusters were scattered differentially throughout the two-dimensional UMAP (td-UMAP) representation of aggregated scRNA-seq data (Fig. 4a). In parallel, viral titration of the remaining brain samples (brain without thalamus which was dissected for CD11b+ cell isolation) showed that each brain exhibited a different viral titer (Additional file 3: Fig. S3; Mouse #1: 57.000 PFUs/μL, Mouse #2: 98.000 PFUs/μL and Mouse #3: 136.000 PFUs/μL). We partitioned these immune cells into the following sub-clusters based on the expression of different genes, but also by taking into account different viral titers promoting different levels of inflammation: two monocytes (Mono1 = Gm34084, Clec12a, Clec4a1; Mono2 = Ms4a8a, Arg1, Chil3), two macrophages (reactive mac = Ccl8, Cbr2, Cd163; homeostatic mac = Pf4, Cd209f, Mrc1), three DCs (dendritic cells) (cDC1 (conventional DC) = Xcr1, Snx22, Sept3; cDC2 = Cacnb3, Nudt17, Ccr7; moDC (monocyte-derived dendritic cell) = (Kmo, Ffar2, H2-DMb2) and four microglia (surveillant microglia = Csmd3, P2ry12, Gpr34; reactive microglia = C4b, Cacna1s, Hspa1b; reactive proliferating microglia = Cenpf, Pcr1, Top2a; “in transition” microglia/microglia-like cells = G0s2, Il1f9, Hdc) (Additional file 3: Fig. S3). Thereafter, another td-UMAP was created to visualize these different cell sub-clusters on the aggregated data (Fig. 4b).
scRNA-seq reveals novel HSE-associated microglia/microglia-like cell transcriptome expressing neutrophil-related genes in highly infected thalamus. Chromium 10X coupled with Illumina sequencing was used to analyze the transcriptome of 2000 CD11b+ immune cells isolated with magnetic beads from the thalamus of the intranasally infected mouse on day 6 p.i. a td-UMAP visualization of aggregated scRNA-seq data, labeled by sample (three infected and two uninfected mice on day 6 p.i.), showing different cell clusters for each sample. b td-UMAP visualization of all sub-clusters labeled with different colors on aggregated scRNA-seq data. c Heatmap showing the top 150 genes whose levels of expression are highest and most differentiating in each of the 17 cell sub-clusters, revealing similar transcriptomic signatures between “in transition” microglia/microglia-like cell and Mono2 subsets. d Violin plots demonstrating differential expression (in log2 fold-change) of 4 neutrophils (Retnlg, Asprv1, Cxcr2, Il1f9) and 4 microglia (Tmem119, Fclrs, Csf1r, P2ry12)-related genes in “in transition” microglia/microglia-like cells, surveillant microglia, reactive microglia, reactive proliferating microglia, Mono1, and Mono2. Statistical analyses were performed on Loupe Browser v5. Statistically, significant results are indicated as follows: ****P < 0.001. e Stacked bar plot showing the mean relative proportion of each cell type in the thalamus of three HSV-1-infected mice (day 6 p.i.) and two uninfected control group (day 0). f Heatmap showing the number of surveillant, reactive, reactive proliferating microglia, “in transition” microglia/microglia-like cells, Mono 1 and Mono2 in three infected samples with different viral titers on day 6 p.i. The minimum and the maximum number of cells (0 and 800 cells, respectively) correspond to red and green colors, respectively
scRNA-seq reveals novel HSE-associated microglia/microglia-like cell transcriptome expressing neutrophil-related genes in highly infected thalamus. Chromium 10X coupled with Illumina sequencing was used to analyze the transcriptome of 2000 CD11b+ immune cells isolated with magnetic beads from the thalamus of the intranasally infected mouse on day 6 p.i. a td-UMAP visualization of aggregated scRNA-seq data, labeled by sample (three infected and two uninfected mice on day 6 p.i.), showing different cell clusters for each sample. b td-UMAP visualization of all sub-clusters labeled with different colors on aggregated scRNA-seq data. c Heatmap showing the top 150 genes whose levels of expression are highest and most differentiating in each of the 17 cell sub-clusters, revealing similar transcriptomic signatures between “in transition” microglia/microglia-like cell and Mono2 subsets. d Violin plots demonstrating differential expression (in log2 fold-change) of 4 neutrophils (Retnlg, Asprv1, Cxcr2, Il1f9) and 4 microglia (Tmem119, Fclrs, Csf1r, P2ry12)-related genes in “in transition” microglia/microglia-like cells, surveillant microglia, reactive microglia, reactive proliferating microglia, Mono1, and Mono2. Statistical analyses were performed on Loupe Browser v5. Statistically, significant results are indicated as follows: ****P < 0.001. e Stacked bar plot showing the mean relative proportion of each cell type in the thalamus of three HSV-1-infected mice (day 6 p.i.) and two uninfected control group (day 0). f Heatmap showing the number of surveillant, reactive, reactive proliferating microglia, “in transition” microglia/microglia-like cells, Mono 1 and Mono2 in three infected samples with different viral titers on day 6 p.i. The minimum and the maximum number of cells (0 and 800 cells, respectively) correspond to red and green colors, respectively
We generated a heatmap of the 150 most significantly up- and down-regulated genes to reveal distinct transcriptomic signatures of all sub-clusters. Overall, our clustering approach successfully distinguished the major cell populations and subpopulations with differential gene expression. As expected, we observed sub-clusters exhibiting similar gene expression patterns. Interestingly, Mono2 and “in transition” microglia/microglia-like cells shared a similar transcriptomic pattern, pointing out a possible differentiation between sub-clusters (Fig. 4c). Further analysis revealed 104 cells highly enriched (log2 fold-change > 8) in neutrophil-related genes. 79 of them belonged to “in transition” microglia/microglia-like cell sub-cluster (Additional file 2: Fig. S2b). Indeed, some well-known genes expressed by neutrophils such as Retnlg, Asprv1, Il1f9, and Cxcr2 were also found in this novel microglia-like transcriptome [53]. We decided to define this cell subtype “microglia-like cells” considering that they highly expressed well-established microglial markers, such as P2ry12, Tmem119, Fcrls, and Csf1r (Fig. 4d). These findings suggest that the “in transition” microglia/microglia-like cell transcriptome whether corresponds to the transcriptomic signature of a new differentiating microglia-like sub-cluster or to microglia/microglia-like cells phagocyting infiltrating neutrophils [54, 55].
The “in transition” microglia/microglia-like cell transcriptome is detected in the highly infected thalamus
We individually analyzed the kinetics of immune cells for each of the five scRNA-seq data sets. Two major clusters of uninfected thalami were microglia and mac, corresponding to 72.7% and 14% of all cell populations detected in those samples, respectively. We also noticed a decrease of microglia/microglia-like cells (72.7% vs. 40.4%, P 0.006) and CNS mac (14% vs. 3.3%, P 0.6529) percentages on day 6 p.i., compared to uninfected brains. By contrast, the levels of infiltrating monocytes significantly increased (0.6% vs. 30.6%, P 0.0073) on day 6 p.i. Monocytes (30.6%), T-cells (17.1%), DCs (4.5%), and NK cells (2.2%) were also present in the infected thalamus across the three examined samples, varying in their viral titers (Fig. 4e). In concordance with our flow cytometry data showing decreased numbers of microglia/microglia-like cells on day 6 p.i. (Fig. 2c), the analysis of cell population kinetics using scRNA-seq data also showed a decreased number of microglia/microglia-like cells on day 6 p.i.
Next, we evaluated the numbers of each microglial and monocytic sub-clusters for three infected samples. 259 surveillant and 369 reactive microglia were found in Mouse #1 sample with the lowest brain viral titer. In the same data set, we identified 115 reactive proliferating microglia and 3 “in transition” microglia/microglia-like cells. Mouse #2, with an intermediate viral titer, had 6 surveillant, 283 reactive, 100 proliferating reactive, and 25 “in transition” microglia/microglia-like cells. Reactive and reactive proliferating microglial sub-clusters, containing, respectively, 97 and 17 cells, continued to decrease with increased viral titers in Mouse #3 sample. The surveillant microglia sub-cluster completely disappeared, and “in transition” microglia/microglia-like cells reached the highest level with 511 cells in the same data set (Fig. 4f).
We observed high levels of infiltrating monocytes in all three infected mice differing in their viral titers. A total of 198, 64, 16 Mono1 and 65, 213, 798 Mono2 cells were detected in Mouse #1, #2, and #3 data sets, respectively. Mono2 levels increased with the severity of the infection (Fig. 4f). We also noticed that their numbers increased along with those of “in transition” microglia/microglia-like cells. This observation raised the question of whether those microglia/microglia-like cells differentiate from other microglial subsets, or Mono2 gives rise to this ambiguous population. Overall, these observations indicate that increased viral titers alter cell phenotypes and promote an increased “in transition” microglia/microglia-like cell transcriptome in the infected thalamus on day 6 p.i.
The “in transition” microglia/microglia-like cell transcriptome indicates a hyperinflammatory microglial phenotype in highly infected thalamic regions
Monocytes and microglia represent hardly distinguishable cell populations in homeostatic conditions. Inflammation-driven phenotypic alterations of these cells in infectious disease models make their identification even more difficult. To distinguish microglial responses, we identified upregulated cluster-specific genes (log2 fold-change > 4; P < 0.05) for infiltrating monocytes, total microglia (surveillant, reactive and reactive proliferating), and “in transition” microglia/microglia-like cells. We included “in transition” microglia/microglia-like cells in this analysis as a major cluster, considering that the origin of these cells is still unknown. The top three most significantly upregulated cluster-specific genes for these three cell populations were: Sirpb1c, Ly6i, Apoc2 for infiltrating monocytes, Csmd3, P2ry12, Slc2a5 for microglia and G0s2, Il1f9, Hdc for “in transition” microglia/microglia-like cells (Fig. 5a (Top 3 genes); Additional file 2: Fig. S2c (Top 50 genes). This analysis allowed the identification of potential biomarkers that could be used to differentiate these clusters.
Analysis of up-regulated pathways uncovers HSV-1-mediated pro-inflammatory response of “in transition” microglia/microglia-like cells during HSE. a Feature plots show the distribution of the top three cluster-specific genes with the highest expression levels on two-dimensional UMAP (td-UMAP) visualization of aggregated data (three infected and two uninfected mice on day 6 p.i.). Csmd3, Slc2a5, P2ry12 for microglia, Sirpb1c, Ly6i, Apoc2 for infiltrating monocytes, and G0s2, Il1f9, Hdc for “in transition” microglia/microglia-like cells. b Homemade heatmap shows increased levels of IFN-stimulated genes involved in antiviral response for “in transition” microglia/microglia-like cells. c Dot plots showing the first 25 most significantly up-regulated Reactome pathways in monocytes and microglial cell sub-clusters. The size of each dot represents the number of genes in each cell sub-cluster, and the color of each dot indicates the normalized enrichment score of each pathway (p-adjust). Pathways that were not significantly enriched (Q value ≥ 0.05, Benjamini–Hochberg correction) are not displayed
Analysis of up-regulated pathways uncovers HSV-1-mediated pro-inflammatory response of “in transition” microglia/microglia-like cells during HSE. a Feature plots show the distribution of the top three cluster-specific genes with the highest expression levels on two-dimensional UMAP (td-UMAP) visualization of aggregated data (three infected and two uninfected mice on day 6 p.i.). Csmd3, Slc2a5, P2ry12 for microglia, Sirpb1c, Ly6i, Apoc2 for infiltrating monocytes, and G0s2, Il1f9, Hdc for “in transition” microglia/microglia-like cells. b Homemade heatmap shows increased levels of IFN-stimulated genes involved in antiviral response for “in transition” microglia/microglia-like cells. c Dot plots showing the first 25 most significantly up-regulated Reactome pathways in monocytes and microglial cell sub-clusters. The size of each dot represents the number of genes in each cell sub-cluster, and the color of each dot indicates the normalized enrichment score of each pathway (p-adjust). Pathways that were not significantly enriched (Q value ≥ 0.05, Benjamini–Hochberg correction) are not displayed
Furthermore, we performed Panther—Gene Ontology (GO) Enrichment Analysis for molecular functions on “in transition” microglia/microglia-like cell cluster, using all significantly up-regulated genes (P < 0.05). The analysis revealed the following upregulated GO terms: “Protein homodimerization activity,” “Chemokine receptor binding,” “Cytokine binding,” “Cytokine activity,” “Carbohydrate derivative binding,” “Nucleotide binding,” “GTPase activity,” and “Enzyme binding.” Interestingly, “double-stranded RNA binding” function, regrouping 6 genes (Ifih1, Adar, Oas2, Oas3, Oasl1, and Oasl2) involved in the antiviral response, including IFN downstream signaling, were identified for this microglial sub-cluster [56,57,58]. Indeed, the assessment of differential expression levels of a homemade gene set demonstrated upregulated expression levels of genes encoding antiviral proteins and IFN-related genes, such as Isg15, Ifi47, Irf7, Ifit1, for the “in transition” microglia/microglia-like cells (Fig. 5b). These results suggest a direct antiviral response orchestrated by “in transition” microglia/microglia-like cells during HSE.
We performed an exploratory study of biological pathways enriched in infiltrating monocytes and microglial sub-clusters to assess cellular functions using the Reactome Knowledgebase (Fig. 5c) (Additional file 4: Fig. S4 Reactome pathway dot plots and enrichment maps for Mono2 and “in transition” microglia/microglia-like cells) (Additional file 5: Fig. S5 Functional heat plots for Reactome pathway gene sets) [59]. Infiltrating Mono1 up-regulated genes associated to metabolic pathways (e.g., Atpc1, Eif3f, Ndufa2), “Antigen processing-Cross presentation (e.g., Cyba, H2-M3, Psma2) and “Immunoregulatory interactions between a Lymphoid and non-Lymphoid cell” (e.g., B2m, H2-T22, Fcgr2) functions, suggesting an increased capacity for antigen processing and presentation (Additional file 4: Fig. S4a, e; Additional file 5: Fig. S5a). Mono2 appeared to be one of the main cytokine/chemokine producers (e.g., CXCL2/10, IL-1α/β, IL-15/18/23a/27) during HSE. We noticed Mono2 activated CLEC7A signaling, which plays a role in the production of TNF, CXCL2, and IL-1β [60]. Amongst several other pathways, “Class I MHC-mediated antigen processing & presentation,” “C-type lectin receptors” (e.g., C3, Clec4n, Fcer1g), and “Fc gamma receptor (FCGR) dependent phagocytosis” (e.g., Actb, Arpc3, Fcgr4, etc.), were up-regulated in Mono2. We also detected upregulation of the “TNFR2 non-canonical NF-KB pathway” (e.g., Birc3, Cd40, Nfkb2) and “Programmed cell death” (e.g., Bax, Bcl2l1, Casp3) for these cells on day 6 p.i. (Fig. 5c, top; Additional file 5: Fig. S5b).
Surveillant microglia were enriched in “Signaling by Receptor Tyrosine Kinases” (e.g., Col27a1, Dusp7, Insr), “Signaling by Rho GTPase” (e.g., Fgd2, Rhoq, Tuba1a), and “Platelet activation, signaling and aggregation” (e.g., Gna12, Mapk14, Pic3cg), “Signaling by VEGF” (e.g., Ncf2, Prkar1a, Rock2) that activates angiogenesis and “Signaling by TGF-beta Receptor Complex” with immunosuppressive functions (Additional file 4: Fig. S4a; Additional file 5: Fig. S5c). In addition, increased expression of the “FCGR dependent phagocytosis” gene set suggested that surveillant microglia exhibit phagocytic activity while maintaining CNS homeostasis. Reactive microglia activated similar RNA and protein metabolic pathways as found in infiltrating Mono1, e.g., rRNA processing (e.g., Ddx21, Gnl3, Nop56), translation (e.g., Pabpc1, Eef1g, Rps8), and non-mediated decay (NMD) (e.g., Rpl11, Rpl12, Rpl14). These cells increased the expression of genes encoding heat–shock proteins (HSPs) (e.g., Hsp90aa1, Hsp90ab1, Hspa1a) involved in the control of oxidative stress, inflammatory response, and antigen presentation [61]. Moreover, Endosomal TLR-mediated recognition of HSV-1 seemed to activate these phagocytes that also play a major role in antigen presentation via the major histocompatibility complex (MHC)-I, but also MHC-II (Additional file 4: Fig. S4c; Additional file 5: Fig. S5d). These results suggest that reactive microglia directly affect CD4+ T cell response [62]. Our results also confirm that microglia proliferate in the CNS upon viral infection. For these reactive proliferating microglia, we identified mitotic cell division pathways, such as “Mitotic Anaphase and Metaphase” (e.g., Banf1, Birc5, Anapc5), “Separation of sister chromatids” (e.g., Bub1, Bub1b, Cdc20), “DNA replication” (e.g., Dbf4, E2f2, Gmnn) and “G2/M checkpoints” (e.g., Cdk1, Exo1, Herc2). We suggest that microgliosis can contribute to the repopulation of these empty niches caused by reactive or dying microglia in the thalamus, although these proliferating microglia exhibiting pro-inflammatory features could also be responsible for the aggravation of the immune response during HSE (Additional file 4: Fig. S4d; Additional file 5: Fig. S5e).
The analysis of “in transition” microglia/microglia-like cells showed that the most significantly enriched pathway was “Interleukin-1 signaling” (e.g., Il1a, Il1b, Il1r2). These microglia/microglia-like cells were activated via multiple TLRs (Tlr2/3/4/6/7). Based on increased expression of Tram, Trif, and the other genes involved in downstream signaling of TLR4, “TRIF-mediated TLR4 signaling” (e.g., Birc3, Cd14, Ikbke) came up as the major pathway. However, we believe that not only TLR4 but also a multitude of TLRs led to the induction of a hyperinflammatory phenotype producing a variety of cytokines/chemokines exacerbating the inflammatory response [63]. This microglial sub-cluster increased the expression of the transcription factor AP-1 (Jun), Interferon Regulatory Factors (Irf1/7/8), as well as other genes (Jak1, Stat1, Stat2, cGas, Tmem173 (Sting), etc.) involved in the IFN-induced JAK–STAT pathway (Fig. 5c, bottom; Additional file 4: Fig. S4f; Additional file 5: Fig. S5f). Our results confirm the up-regulation of the “Interferon signaling” (e.g., Eif2ak2, RnaseI, Socs3) pathway, suggesting that these microglia/microglia-like cells play a major role in viral detection and type I IFN production.
“ER-Phagosome pathways” (e.g., H2-Q10, H2-T23, B2m) and “Antigen-presentation via MHC-I” (e.g., H2-Q6, Tap1, Tap2) were activated in “in transition” microglia/microglia-like cells, as well as in reactive microglia and Mono2. Contrarily to other microglia and monocyte sub-clusters, antiviral pathways such as “Antiviral mechanism by IFN-stimulated genes” (e.g., Arih1, Usp18, Oasl1), “DDX58/IFIH1-mediated induction of interferon-alpha/beta” (e.g., Nfkb1, Nfkb2, Tax1bp1), and “ISG15 antiviral mechanism” (e.g., Isg15, Trim25, Ube2l6) were present in the pathway list of “in transition” microglia/microglia-like cells (Fig. 5c). They overexpressed dsRNA helicase genes [e.g., Mda5 (Ifih), Lgp2 (Dhx58)], Interferon Stimulated Exonuclease Genes (e.g., Isg15 and Isg20) and other genes encoding antiviral proteins like Acod1 (Aconitate Decarboxylase 1), Ifit1 (Interferon-induced protein with tetratricopeptide repeats 1 Gene), Rsad2 (radical SAM domain-containing, also known as Viperin) and Hcar2 (Hydroxycarboxylic Acid Receptor 2) [57, 64,65,66,67,68]. Based on these highly expressed cytoplasmic antiviral factors, we hypothesize that “in transition” microglia/microglia-like cells with hyperinflammatory features correspond to HSV-1-infected microglia attempting to inhibit viral replication.
We also observed significant enrichment of programmed cell death pathways, including “RIPK1-mediated regulated necrosis” (e.g., Cflar, Fas, Mlkl), “Regulated Necrosis” (e.g., Peli1, Ripk1, Sdcbp), “Regulation of necroptotic cell death” (e.g., Birc3, Casp8, Ube2l6), for “in transition” microglia/microglia-like cells. In parallel, overexpression of genes (e.g., Ripk3, Fas, Tnf, Tnfsf10, Nlrp3) involved in TNF and NLR signaling were observed. Overexpressed “casp8 activity is inhibited” in “in transition” microglia/microglia-like cells, suggesting that the blockage of CASP8 activity in the presence of viral FLIP-like protein switches apoptotic signaling to necrotic cell death [69]. In addition, higher expression of Cflar (cellular FADD-like interleukin-1 beta converting enzyme (FLICE)-inhibitory protein (cFLIP, encoded by the Cflar)) inhibiting apoptosis and promoting necroptosis, strengthens the possibility that “in transition” microglia/microglia-like cells undergo necroptosis.
The “in transition” microglia/microglia-like cell transcriptome corresponds to the antiviral response of HSV-1-infected microglia
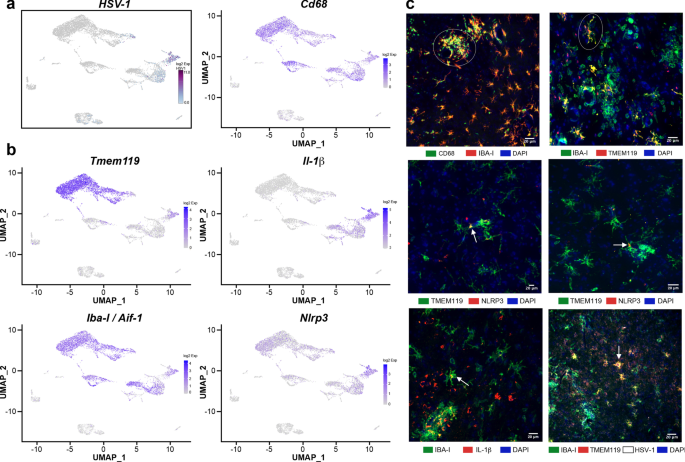
To clarify whether “in transition” microglia/microglia-like cells phagocytose-infected cells or represent HSV-1-infected microglia, we searched for reads corresponding to the viral transcripts in our scRNA-seq data as described previously [70]. Our data showed that few Mono1 and T cells contained viral mRNA. We noticed that Mono2 was highly enriched in viral transcripts. Of note, “in transition” microglia/microglia-like cells exhibited the most elevated levels of HSV-1 transcripts (log2 fold-change > 5, Fig. 6a). We suggest that similar transcriptomes of “in transition” microglia/microglia-like cells and Mono2 could result from HSV-1 infection activating similar antiviral mechanisms. Altogether, such high levels of viral transcripts suggest a potential viral replication phase for these two clusters.
In situ characterization of “in transition” microglia/microglia-like cells in HSV-1-infected VPLs. a Distribution of HSV-1 transcripts was analyzed to identify infected cell sub-clusters on two-dimensional UMAP (td-UMAP) visualization of aggregated data (three infected and two uninfected mice on day 6 p.i.). “In transition” microglia/microglia-like cell sub-cluster corresponds to HSV-1-infected microglia/microglial-like cells. b Feature plots show the distribution of Tmem119, Iba-I (Aif1), CD68, Nlrp3, and Il-1β genes with on td-UMAP of aggregated data. Available antibodies against the proteins encoded by canonical genes were used to identify “in transition” microglia/microglia-like cells. c (top, left) IBA-I+ cells expressing high levels of CD68 clustered (white circle) in highly infected thalamus. (top, right) TMEM119 and IBA-I immunostaining revealed ramified TMEM119+/IBA-I+ cells (white circle) near clusters of TMEM119−/IBA-I+ cells in infected thalamus on day 6 p.i. Inflammasome activity in “in transition” microglia/microglia-like cells was studied using NLRP3 (middle, left, and right) and IL-1β (bottom, left) immunostaining on HSV-1+ VPLs. Confocal microscopy images revealed TMEM119+/NLRP3+ and IBA-I+/IL-1β + microglia/microglia-like cell populations (white arrow) highlighting “in transition” microglia/microglia-like cells. (bottom, right) Immunohistochemical staining for HSV-1 showed that these ramified TMEM119+/IBA-I+ cells were HSV-1+ (white arrow) (scale, 20 μm)
In situ characterization of “in transition” microglia/microglia-like cells in HSV-1-infected VPLs. a Distribution of HSV-1 transcripts was analyzed to identify infected cell sub-clusters on two-dimensional UMAP (td-UMAP) visualization of aggregated data (three infected and two uninfected mice on day 6 p.i.). “In transition” microglia/microglia-like cell sub-cluster corresponds to HSV-1-infected microglia/microglial-like cells. b Feature plots show the distribution of Tmem119, Iba-I (Aif1), CD68, Nlrp3, and Il-1β genes with on td-UMAP of aggregated data. Available antibodies against the proteins encoded by canonical genes were used to identify “in transition” microglia/microglia-like cells. c (top, left) IBA-I+ cells expressing high levels of CD68 clustered (white circle) in highly infected thalamus. (top, right) TMEM119 and IBA-I immunostaining revealed ramified TMEM119+/IBA-I+ cells (white circle) near clusters of TMEM119−/IBA-I+ cells in infected thalamus on day 6 p.i. Inflammasome activity in “in transition” microglia/microglia-like cells was studied using NLRP3 (middle, left, and right) and IL-1β (bottom, left) immunostaining on HSV-1+ VPLs. Confocal microscopy images revealed TMEM119+/NLRP3+ and IBA-I+/IL-1β + microglia/microglia-like cell populations (white arrow) highlighting “in transition” microglia/microglia-like cells. (bottom, right) Immunohistochemical staining for HSV-1 showed that these ramified TMEM119+/IBA-I+ cells were HSV-1+ (white arrow) (scale, 20 μm)