Diagnosis of animal trypanosomoses: proper use of current tools and future prospects | Parasites & Vectors | Full Text
There are critical periods in the course of an infection that are directly associated with the reliability of a diagnosis. For instance, at the onset of an infection, during the first 2–3 weeks after a Trypanosoma infection, any samples analysed by parasite or antibody detection methods may provide false-negative results. Likewise, parasite detection tests in asymptomatic carriers may not reveal the infection, while antibody detection tests would in this case be effective. Conversely, after curative treatment, it may not be possible to ascertain the presence or absence of infection due to the persistence of antibodies in the serum for months [7]. Serial samplings may address such situations.
One or more diagnostic tools need to be used during single or serial sampling to enable a conclusion to be drawn or inference made on a mammal’s status regarding trypanosome infection, whether “non-infected” or “actively infected”, which can either be an “asymptomatic carrier” or a “sick carrier”. As was previously discussed [3], the test specificity can vary, the primers must be selected according to subgenus, species, type and subspecies, and the test results will still remain inconclusive for single or mixed infection status. By using one or more diagnostic tools for serial examinations, it will be possible to differentiate current (parasite ± antibodies) from past infections (antibodies only). Possible outcomes of diagnostic tests regarding the infection and immune statuses are given in Fig. 1.
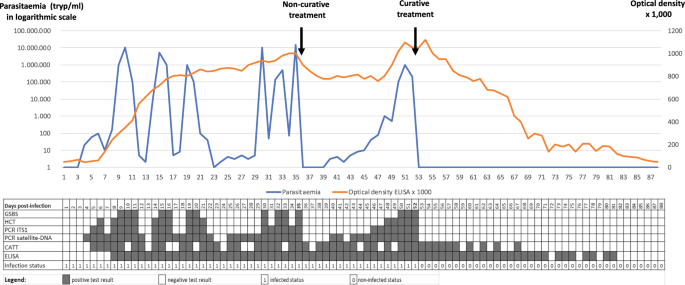
Parasitaemia in trypanosomes per millilitre (tryp/ml; blue curve) and optical density × 1000 in ELISA (orange curve), modelled in an animal infected by a trypanosome (Trypanosoma evansi in this case) on D0 (Day 0), receiving 1 non-curative treatment on D35, and one curative treatment on D52. See Abbreviation List for the full description of each abbreviation
Parasitaemia in trypanosomes per millilitre (tryp/ml; blue curve) and optical density × 1000 in ELISA (orange curve), modelled in an animal infected by a trypanosome (Trypanosoma evansi in this case) on D0 (Day 0), receiving 1 non-curative treatment on D35, and one curative treatment on D52. See Abbreviation List for the full description of each abbreviation
This figure represents test outcomes from infected animals (T. evansi in cattle, for example) receiving non-curative and curative treatments. Based on the results gathered during experimental infections, we modelled the follow-up of an animal infected on day 0 (D0). In this model, the positive thresholds for the diagnostic techniques were set up as follows: Giemsa-stained thin blood smear (GSBS): 10,000 trypanosomes per millilitre (tryp/ml); haematocrit centrifugation technique (HCT): 100 tryp/ml; PCR-internal transcribed spacer 1 (ITS1): 30 tryp/ml; satellite-DNA PCR: 3 tryp/mll, and enzyme-linked immunosorbent assay (ELISA) optical density (OD) = 0.2. A non-curative treatment was given on D35; this was followed by an “aparasitaemic” period, but the OD in ELISAs remained high. A relapse of the infection was detected from D40 onwards by satellite-DNA PCR, followed by PCR-ITS1 (D46), HCT (D48) and GSBS (D50). A curative treatment on D52 was followed by an “aparasitaemic” period then a progressive decrease, based on OD, until it became negative from D82 onwards. A negative seroconversion 30 days after curative treatment is short; longer periods are generally observed in the field, particularly in older animals having undergone multiple infections. The inconsistency in detection of early infections by the card agglutination test for trypanosomosis (CATT) can be attributed to the presence of immune complexes [8]. On the other hand, it turns negative before the ELISA due to the short half-life of immunoglobulin M (IgM).
Establishment of a “non-infected” status
Due to fluctuating parasitaemia, it is not always possible to demonstrate the presence of trypanosomes in infected animals. Therefore, negative results from parasitological and/or molecular techniques are insufficient to establish a “non-infected” status. Repeated non-detection of antibodies after 1 month (> estimated incubation period of 2–3 weeks) is a stronger criterion.
Immunoglobulin G (IgG) produced against trypanosomes can be detected in the serum from 2 to 3 weeks after infection. According to the recommendations of the World Organisation for Animal Health (WOAH, formerly known as OIE), this is especially true with ELISA plates coated with a complete native antigen, such as whole-cell lysate-soluble antigens (WCLSA) [9, 10]. IgG detection is thus a reliable method for establishing a “non-infected” status in most cases. However, negative parasitological, molecular and serological results are needed twice at a 1-month interval [10].
Nonetheless, a serological test may give a false-negative result in the case of Trypanozoon infections that display occasional extravascular foci, whereby the parasite is not in contact with the immune system [11]. This was hypothesised in the T. evansi camel outbreak that occurred in France [1]. However, such a situation is probably rare and limited to T. evansi in camels. In most cases, accurate indications are obtained from an ELISA, as demonstrated in several validations among different host species. All of these studies agreed on the high sensitivity and reliability of IgG detection by ELISA using WCLSA of trypanosomes [12,13,14,15,16,17,18,19].
In conclusion, with the exception of surra in camels, an unequivocal “non-infected” status can be established (in cattle, buffaloes, horses, sheep, goats, dogs, etc.) if negative results are obtained in a quarantine context, twice at a 1-month interval [9, 10], using: (i) HCT; (ii) one or more molecular detection tests (PCR) selecting the most suitable primers; and (iii) one or more WCLSA ELISA(s), selecting the most suitable species (ELISA T. vivax, T. congolense, T. brucei/T. evansi and/or T. cruzi in Latin America), according to the geographical area and the epizootiological situation [3]. Furthermore, due to its cross-reactivity with all pathogenic mammalian trypanosomes (T. brucei, T. equiperdum, T. vivax, T. congolense and T. cruzi), the T. evansi ELISA test would be an ideal candidate for such screening [17, 20,21,22].
In animals for which no species-specific anti-IgG conjugate is commercially available, protein A-conjugate may be used. However, results using this conjugate have not been fully validated and standardised due to a lack of reference sera from non-infected and infected animals. Consequently, the status of such animals regarding trypanosomosis cannot be certified.
Detection of an “active infection”
Microscopic examinations of GSBSs and buffy coat [23] are quick and cheap ways of detecting active infections. These methods remain the most usual choice in enzootic areas. However, they lack sensitivity and require a minimal level of equipment and skill, which are not always available in the field. Nevertheless, a positive result indicates a parasitaemia > 50–100 tryp/ml, which reflects the immune system’s inability to control the infection and should lead to a treatment decision.
Other parasitological methods, such as the kit for in vitro isolation (KIVI) [24] or the mouse inoculation technique (MIT) (although raising ethical issues), remain the most efficient techniques for parasite isolation [8]. They can be used to demonstrate the parasite’s presence, but also allow further characterisation and storage of field isolates. When applied to diagnosis, they are more sensitive than HCT and GSBS [25], but are relatively expensive and time-consuming. Still, they are helpful for trypanosome isolation during an outbreak in a previously non-endemic area [26] or for high-value animals (racehorses, zoo animals, etc.). The added value of the MIT is clear for Trypanosoma species such as T. evansi and T. brucei that multiply readily in rodents, but for other Trypanosoma species, such as T. congolense and T. vivax, results are inconsistent and, in general, negative for T. equiperdum [27].
Molecular detection of trypanosomes through PCR was a real breakthrough in the development of trypanosome diagnostic techniques in the 1990s [28,29,30]. PCR improved the sensitivity for detecting active infections and significantly improved specificity, at various taxonomic levels. However, molecular methods have critical limitations: (i) they leave cases with low parasitaemia or non-circulating parasites undetected [31, 32]; (ii) they are limited to fully equipped laboratories with skilled technicians; (iii) positive results are conclusive of active infection (leaving aside the fact that DNA may still be detected 24–48 h after curative treatment [33]), but negative results are not; and (iv) there is a significant delay between the time of sampling in the laboratory and the delivery of results, so animals can be out of reach by the time the veterinarian, vet technician or owner receives the results. Loop-mediated isothermal amplification methods (LAMP) applied to parasite DNA can mitigate such drawbacks. Although they were claimed to be efficient and applicable in the field [34,35,36], this has never really been the case. The new polymerase spiral reaction (PSR) method [37] may be suitable for field diagnosis in real time, but it still requires comprehensive field validation. Finally, the new and promising spliced-leader RNA (SL-RNA) detection method is applied to a short and conserved RNA sequence linked to the 5’ end of each trypanosome pre-messenger RNA (mRNA) [38]. Still, its implementation requires expensive equipment for quantitative PCR (qPCR) and skilled personnel [39].
Although antibody detection indicates contact between the host and parasites, it does not confirm active infection, especially as IgG persists several weeks after treatment or self-cure (2–4 months). IgM is produced early and has a short half-life (1–3 months) [8, 40], and is associated with recent infection or recent parasite circulation. IgM detection has a good positive predictive value for detecting active infections, while IgG tests detect an “established infection”. However, IgM immune complexes are captured by phagocytic cells in the serum of actively infected animals and, consequently, IgM detection can give false-negative results [8]. Overall, implementing IgM and IgG detection in a herd showing signs of active infection (positive HCT or PCR) can help identify infected animals, but these methods not suited to detect active infection on their own.
In summary, at the individual level, an active infection can only be established with parasitological (HCT, GSBS, MIT, etc.) and/or molecular tools (PCR, LAMP, PSR, RNA detection, etc.). However, once the infection is confirmed in one or more animals in a group, seropositivity may be considered sufficient by a primary care veterinarian to decide on eliminating parasites, even in apparently healthy animals. Conversely, a “sickness treatment-decision strategy” requires evidence that clinical signs are linked to active infection (see following sections).
Sick or healthy status and treatment decisions
As discussed earlier [3], in enzootic areas (and in the absence of an elimination programme), before deciding on a treatment, a distinction should be made between the “infected and healthy animal” (asymptomatic carrier) and “infected and sick animal” statuses. A meta-analysis aggregation including averaging the results of 180 studies on cattle trypanosomosis in 19 enzootic African countries [41] showed a low prevalence of 15.1% (95% confidence interval: 13.2–17.1). Nevertheless, on a smaller scale, in enzootic areas of Burkina Faso, Cameroon and Ghana, > 50–70% of the cattle are seropositive [42,43,44]. In such areas, a high percentage of cattle are asymptomatic carriers. Therefore, unless there is an ongoing disease elimination programme, despite being seropositive, these animals do not need treatment. A high seropositivity level makes it difficult (if different from tossing a coin) to draw a causal relationship between the presence of anti-trypanosome antibodies and sickness due to trypanosome(s), even in animals with clinical signs compatible with trypanosomosis and other diseases [45]. There are no markers of “trypanosome sickness”. Although a low haematocrit value can help, the sickness may be caused by other agents, such as ticks, Haemonchus spp., haemoparasites, etc. [46]. HCT is an excellent indicator because its low sensitivity indicates a medium to high parasitaemia, suggestive of “insufficient immune control”. Additionally, it may estimate anaemia (low packed-cell volume), which is undoubtedly sufficient reason for trypanocidal treatment.
As microscopes and centrifuges are rarely available in the field, a rapid antigen detection test would help evidence recent circulation of parasites, even with limited sensitivity. Like the T. evansi CATT, card agglutination tests for other Trypanosoma spp., based on IgM, would be of predictive value for trypanosome sickness and support treatment decisions.
Can species and/or subspecies-specific diagnosis be established?
The ELISA offers a large panel of antigens with sensitivity close to or higher than 95% [15, 47, 48] and a high specificity in relation to other genera, such as Anaplasma, Babesia, etc. However, species specificity remains low and strong cross-reactions occur between the main animal trypanosomoses of African origin (ATAO): T. vivax, T. congolense, T. brucei brucei, T. evansi and T. equiperdum [17, 49]. These cross-reactions are due to common antigens shared by salivarian Trypanosoma, but even occur between taxonomically distant species such as T. evansi and T. cruzi [20], or Leishmania [20, 50, 51]. The species specificity of serological tools and therfore “seropositivity” is thus questionable. Fortunately, Megatrypanum such as T. theileri has been shown not to cross-react in an ELISA for trypanosomes [52, 53]. Consequently, ELISAs carried out with WCLSAs of salivarian trypanosomes are specific to “pathogenic Trypanosoma spp.”, but fail to identify them at the species level.
For ATAO control, in most cases−and especially in tsetse-infested areas−a species-specific diagnosis may not be necessary because the control tools (e.g. fly traps and trypanocides) are mostly identical, regardless of the salivarian Trypanosoma species involved. However, knowledge of the infecting Trypanosoma spp. is useful for adjusting the dose and trypanocide to be used. For example: (i) if T. evansi is identified, melarsomine hydrochloride is preferable; (ii) in a nagana area, identifying the species would allow the dose of diminazene aceturate to be adapted to 7 mg/kg for a Trypanozoon infection versus 3.5 mg/kg for an infection by T. vivax or T. congolense.
At the genus level, the co-infection status of an animal seropositive for Trypanosoma spp. or Leishmania spp. antibodies cannot be established using immunodiagnosis due to cross-reactions. In Latin America, cross-reactions should be suspected in studies involving Trypanosoma spp. (T. evansi, T. vivax, T. cruzi, T. equiperdum) and Leishmania spp. Similarly, caution is required with Leishmania and Trypanosoma serological studies in Asia and Africa. Indeed, in Latin America for example, T. vivax and T. evansi ELISAs may react or cross-react due to infection(s) by T. vivax, T. evansi and/or T. cruzi. This is especially so for horses and pigs when using a T. evansi ELISA [54, 55], but also for cattle [56] and buffaloes for both tests [57]. Additionally, Leishmania infections, which may be prevalent in reservoirs such as dogs, are sources of interference [58]. In such cases, the use of species-specific molecular tests and point-of-care diagnostics (POCD) such as recombinant polymerase amplification with lateral flow dipstick (RPA-LFD), recently developed in Mexico for T. cruzi, would be of great value [59]. Furthermore, the recent discovery of Trypanosoma caninum complicates the diagnosis of Trypanosomatidae infections in dogs [60, 61]. In the USA, where T. cruzi [62] and Leishmania [63] are prevalent in horses, interference in serological diagnosis should be suspected, and this situation may also occur in dogs [64].
In Africa, unlike the agents causing nagana, which are considered to be a unique complex entity (at least for their control), species-specific diagnosis may be needed when human pathogens are circulating in animals. It is essential to investigate animal reservoirs to control and eliminate human pathogens [65]. Molecular techniques are well suited for this purpose since they are sensitive and specific. However, PCR may fail to detect infection when primers target a single-copy gene or when using a sample with low parasitaemia. Nested-PCR or RNA detection methods can increase the sensitivity of these tests. Identifying T. b. gambiense and T. b. rhodesiense using subspecies-specific antigen detection would be another option. However, although precise tests may be developed, they would probably have low sensitivity (due to the inverse relationship between sensitivity and specificity), leading to uncertainty when diagnosing negative test results.
Effective and user-friendly tests able to distinguish between all parasites of the subgenus Trypanozoon at the species or subspecies level [66] either have not yet been developed or lack sensitivity. For example, tests to distinguish T. evansi (type A, type B, etc.) from T. equiperdum (type OVI, BoTat, etc.) are inconclusive because of polyphyly [66,67,68,69] or the low sensitivity of the method, which uses single-gene DNA detection tests. In addition, variations in mitochondrial DNA (mtDNA) content (kinetoplastic DNA composed of maxi- and minicircles) can be used to differentiate some Trypanozoon species [70, 71], but the fact that many T. evansi strains are deficient in kinetoplastic DNA (akinetoplastic) [72] limits the widespread use of these tools. Whichever serological test is used (CATT for T. evansi or any of the Trypanozoon ELISAs), in areas of mixed infections, when a positive result suggests a subgenus Trypanozoon infection, it should only be considered a “pathogenic trypanosome infection”. Trypanozoon identification at the subspecies level is possible with DNA-based methods, but at the expense of sensitivity. As an example, positive results are obtained with satellite DNA detection (TBR/NRP primers), which is highly sensitive for Trypanozoon parasites [28, 30] thanks to the 10,000–20,000 sequence repeats. However, specific primers targeting single genes like SRA (T. b. rhodesiense/T. b. brucei) or TGSGP (T. b. gambiense/T. b. brucei) [73, 74]) may be ineffective because of insufficient DNA in the sample. Therefore, when TBR primers provide positive results, the final result will remain inconclusive if single-gene PCRs are negative. PCR sensitivity for diagnosis can thus be ranked as follows: satellite DNA > moderately repeated genes (e.g. ribosomal DNA, ITS1) > single-gene DNA.
When using PCR, a positive test is considered conclusive and a negative one is not, so when the taxon Trypanozoon is detected in a sample, even if one subtaxon is confirmed (e.g. T. evansi using Rode Trypanozoon antigen-type [RoTat]1.2 primers), it will not be possible to detect another Trypanozoon (namely in this example T. equiperdum, T. b. brucei, T. b. rhodesiense or T. b. gambiense) also present in the sample. Thus, a reliable and specific species/subspecies diagnosis may never be established in mixed enzootic hosts and areas.
In conclusion, we urgently need reliable species-specific methods to detect trypanosome infections because of: (i) cross-reactions in antibody detection methods; and (ii) the poor sensitivity of current highly specific DNA-based methods. Even though the epizootiological context would suggest the most probable conclusion (e.g. a trypanosome infection in a horse in the USA is likely to be caused by T. cruzi, while in Asia it would most probably be due to T. evansi), reasonable assumptions may be made only in enzootic areas. Even so, these conclusions cannot be drawn for travelling animals, which could have been exposed to “out of context” parasites. Let us give some rather extreme but also realistic examples. A dog or a racehorse from Africa, which has spent some days in Mexico and then has tested seropositive to an ELISA for T. brucei upon arrival in France should be considered as a potential carrier of one or more of the following pathogens: T. vivax, T. congolense, T. brucei spp., T. evansi, T. equiperdum (for the horse only), T. cruzi and Leishmania spp. [17, 20]. If the same animal has positive PCR results for Trypanozoon, this result confirms it as a carrier of T. brucei spp. and/or T. evansi and/or T. equiperdum. At the same time, it remains suspect for all the other taxa mentioned above due to the PCR’s limited sensitivity.
Although monospecific infections remain the most frequent (thus explaining why we qualified our examples as “extreme”!), to be precise and exhaustive, we must keep in mind the possibility of combined infections. Thus, the only short conclusion on the lack of specificity and sensitivity in trypanosome diagnosis is that once a pathogenic Trypanosoma (or Leishmania) infection is detected, it can hide co-infection(s) by any other pathogenic Trypanosoma (or Leishmania). Such an animal is then a confirmed case of a Trypanosoma infection, in addition to a suspected case of other infection(s) by members of the Trypanosomatidae family.
Diagnosis of trypanosomes in insect vectors
Detecting trypanosomes in insect vectors is feasible for epidemiological studies and risk assessment, but the meanings and limits of any conclusion need to be further discussed.
Trypanosomes can be detected in the mouthparts, salivary glands, midguts, rectum or crushed whole insects, with different significance in terms of development and transmission (mature or immature stage of the cyclical development).
The microscopic observation of trypanosomes in insects does not allow identification of the species since the morphology of insect stages of trypanosomes is not characteristic; therefore, pathogenic trypanosomes may be confused with non-pathogenic ones such as T. theileri (cyclically transmitted by tabanids), or with insect commensals of the Trypanosomatidae family, such as Crithidia [75].
In mechanical vectors
Detecting trypanosomes in the mouthparts of a mechanical vector suggests only a potential for transmission since they can only survive there briefly (30 min to 2 h) [76, 77]. Consequently, detecting trypanosomes in the mouthparts or the midgut of a mechanical vector indicates that the insect fed on an infected animal, but not that the parasite was transmissible to a host. Such information is not particularly relevant; it is easier and more informative to detect trypanosomes in the blood of individual livestock than in blood that is “randomly collected” by an insect. Detecting trypanosomes in haematophagous insects acting as mechanical vectors is therefore of little practical value on livestock farms [75].
However, the situation is different in conservation areas. Since wild animals are difficult to trap or are subject to regulations forbidding their capture, the blood collected by haematophagous insects can be of great interest. Indeed, the detection of trypanosomes in mechanical vectors feeding on wild fauna will provide information on the presence and circulation of these parasites among wild animals and their potential role as reservoirs. In such cases, identifying the insect’s blood meal could provide other complementary information using ELISA [78] or molecular methods, such as PCR amplification of the cytochrome b mtDNA [79]. In addition, it is possible to obtain more information on the vertebrate host through molecular markers or using more recent multiplexed next-generation sequencing (NGS) methods, such as metabarcoding [80, 81]. The application of such methods would notably help to determine the potential circulation of the parasites between host species. However, detection of this kind is rarely implemented due to the low rate of fed insects entering insect traps. This rate could be increased through the use of ultraviolet (UV) light [82], although cost remains a limiting factor.
In cyclical vectors
The case of cyclical vectors of trypanosomes is quite different. When these insects (tsetse flies or triatomine bugs) are found to be “infected” by trypanosomes, the probability that they transmit the parasite is high because they remain permanently infective; their role in the disease epidemiology can thus be addressed and measured [83].
Parasitological methods were the primary tool in the past. They are based on the location of parasites in the vector (gut, salivary glands, proboscis, salivary secretion, faeces) rather than on parasite morphology, which is inconsistent at the insect stages [84]. Molecular techniques have since proved to be more reliable and valuable than parasitological methods for epidemiological studies [85], but contamination during insect dissection could affect detection. Molecular methods are also expensive, especially when considering the total number of PCR tests required per insect, as the number of insect organs is multiplied by the number of Trypanosoma species investigated [85]: 3 × 3 = 9 for (gut + salivary glands + proboscis) × (T. vivax + T. congolense + T. brucei), for example. One option is to dissect the insect and observe body parts through a microscope first, then proceed with PCR tests only for positive samples. Such methods were used to study T. cruzi circulation in Latin America in the framework of large-scale studies to identify biological, ecological and environmental variables associated with Chagas disease [86]. In Africa, they were used to identify pathogenic trypanosomes in tsetse flies [87].
Xenodiagnosis, previously used only for humans [88], is an exciting avenue to explore; it is a sensitive and specific tool to identify trypanosomes in animal and human hosts. Unfortunately, although used for Leishmania [89] and T. cruzi detection in Latin America [90], it has been limited to experimental infections for African trypanosomes [91].
In conclusion, the benefit gained from trypanosome detection in a mechanical vector is limited to wild animals or the interface between livestock and wildlife. More general information can be obtained from tsetse flies and triatomine bugs. Better information should be obtained when combining molecular identification and blood meal analyses [83, 92]. However, the cost of such studies is high since they require insect capture, identification and dissection, (multi-organ) × (multi-species) PCR diagnosis and multi-host blood meal identification [93].