Allergic reactions to tick saliva components in zebrafish model | Parasites & Vectors | Full Text
Experiments in zebrafish were conducted in strict accordance with the recommendations of the European Guide for the Care and Use of Laboratory Animals. Fish were housed and experiments were conducted at an experimental facility (Catalonia Institute for Energy Research [IREC], Ciudad Real, Spain) with the approval and supervision of the Ethics Committee on Animal Experimentation of the University of Castilla La Mancha (PR-2021-09-14) and the Department of Agriculture, Environment and Rural Development of Castilla La Mancha (REGA code ES130340000218).
Experimental design
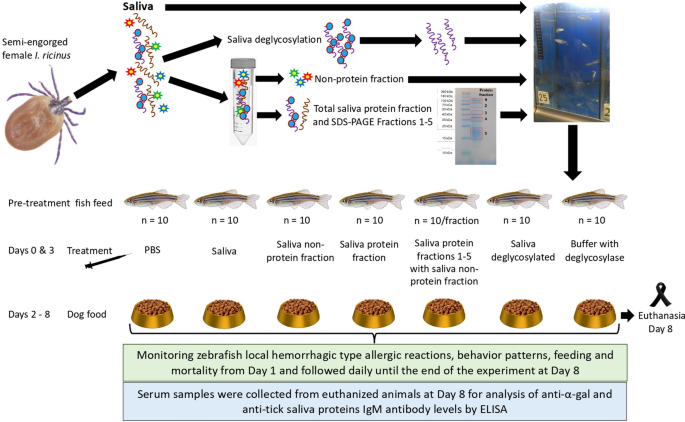
The experiment was designed to characterize tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of AGS (Fig. 1, Ref. [24]) Saliva from semi-engorged I. ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated fractions. The α-Gal content was quantified in tick saliva in comparison with pig kidney (positive control) and human Caucasian promyelocytic leukemia HL60 cells (negative control) as described previously [24]. Protein content was quantified in tick saliva and its fractions used for treatment of zebrafish (Fig. 2A). The amount of protein administered by fish is shown in Fig. 2A. PBS and buffer with deglycosylase were used as negative controls. Wild-type adult [6–8-month-old) AB strain zebrafish (10 animals per group; 1:1 female-to-male ratio; 330 ± 70 mg weight) were kept on fish feed during pretreatment and until day 2. At days 0 and 3, zebrafish were intramuscularly injected with each treatment, and from day 2 until the end of the experiment at day 8 fish were fed dog food containing mammalian meat. Zebrafish hemorrhagic type allergic reactions (skin redness), behavior (abnormal behavior patterns and abnormal or no feeding), and cumulative mortality were examined throughout the experiment and compared between groups to assess the effect of treatments and dog food after feed change and treatment between days 1 and 7 as reported previously [24]. After fish euthanasia, serum was collected from each animal to determine anti-α-Gal and anti-tick saliva protein IgM antibody titers equivalent to human IgE/IgG antibodies [32]. Kidney and intestine samples were collected from euthanized animals at day 8 and stored at −80 °C for further analysis.
Experimental design to characterize tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of alpha-Gal syndrome (AGS). Saliva from semi-engorged Ixodes ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated saliva fractions. Tick saliva fractions with quantified protein content were used for treatment of zebrafish. PBS and buffer with deglycosylase were used as negative controls. Wild-type adult AB strain zebrafish (10 animals per group; 1:1 female to male ratio) were kept on fish feed during pretreatment and until day 2. Zebrafish were injected with each treatment at days 0 and 3, and from day 2 until the end of the experiment at day 8 fish were fed dog food containing mammalian meat. Zebrafish hemorrhagic type allergic reactions, abnormal behavior patterns and abnormal or no feeding, and cumulative mortality were examined after feed change and treatment at day 3 and followed daily until the end of the experiment at day 8. After fish euthanasia, serum was collected from each animal to determine anti-α-Gal and anti-tick saliva protein IgM antibody titers
Experimental design to characterize tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of alpha-Gal syndrome (AGS). Saliva from semi-engorged Ixodes ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated saliva fractions. Tick saliva fractions with quantified protein content were used for treatment of zebrafish. PBS and buffer with deglycosylase were used as negative controls. Wild-type adult AB strain zebrafish (10 animals per group; 1:1 female to male ratio) were kept on fish feed during pretreatment and until day 2. Zebrafish were injected with each treatment at days 0 and 3, and from day 2 until the end of the experiment at day 8 fish were fed dog food containing mammalian meat. Zebrafish hemorrhagic type allergic reactions, abnormal behavior patterns and abnormal or no feeding, and cumulative mortality were examined after feed change and treatment at day 3 and followed daily until the end of the experiment at day 8. After fish euthanasia, serum was collected from each animal to determine anti-α-Gal and anti-tick saliva protein IgM antibody titers
Protein and α-Gal content in tick saliva components and fractions. A Saliva from semi-engorged Ixodes ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated saliva fractions. Protein content was quantified in tick saliva fractions used for treatment of zebrafish. B The α-Gal content was quantified by ELISA in tick saliva and tick saliva protein, non-protein, and deglycosylated components in comparison with pig kidney (positive control) and human Caucasian promyelocytic leukemia HL60 cells (negative control). The quantitation of α-Gal content was performed twice, with similar results
Pathogen-free I. ricinus ticks were obtained from the laboratory colony maintained at the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences of the Czech Republic (IPBCAS), in České Budějovice. All animal experiments were performed in accordance with the Animal Protection Law of the Czech Republic no. 246/1992 Sb (ethics approval no. 34/2018). Semi-engorged female ticks fed for 6–7 days on guinea pigs were inoculated into the hemocoel with 5 μl of a 2% (w/v) solution of pilocarpine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) in PBS, and approximately 0.6 μl of saliva was collected per tick as described previously [24, 33]. Saliva was then transported and stored at −80 °C until use.
Tick saliva protein and non-protein fractions
Tick saliva (135 µl) was diluted 1:1 in PBS, and 255 µl was filtered twice through an Amicon 3 kDa unit (Merck & Co., Inc., Kenilworth, NJ, USA). Of this, 200 µl passed through the Amicon membrane and was considered the non-protein fraction. The 50 µl that did not pass through the Amicon membrane was considered the protein fraction.
Glycosidase treatment of tick saliva
For protein deglycosylation, 20 µl of tick saliva was incubated under denaturing conditions with a cocktail of α-Gal-free glycosidases (PNGase F, 36 kDa; α-(2-3,6,8,9)-neuraminidase, 69 kDa; O-glycosidase, 180 kDa; β(1-4)-galactosidase, 350 kDa; β-N-acetylglucosaminidase, 140 kDa) that removes both asparagine-linked (N-linked) and serine/threonine-linked (O-linked) oligosaccharides using the EDEGLY enzymatic protein deglycosylation kit (Merck & Co., Inc.) and following the manufacturer’s recommendations [34]. After deglycosylation, the tick saliva sample was diluted 1:20 in PBS for filtration first through an Amicon 50 kDa unit (Merck & Co., Inc.) to remove deglycosylases except PNGase F, and then the flow-through with tick salivary proteins (most proteins had less than 50 kDa; Fig. 2A) was filtered through the Amicon 3 kDa (Merck & Co., Inc.) to remove buffer and retain proteins.
Tick saliva protein fractionation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and characterization by mass spectrometry analysis
To obtain the different tick saliva protein fractions, 70 µg from the saliva protein fraction was mixed in 1:1 proportion with Laemmli sample buffer and applied onto two 1.2-cm-wide wells on two 10% SDS-PAGE gels. In one gel, protein bands were visualized by staining with GelCode Blue Stain Reagent (Thermo Fisher Scientific, Waltham, MA, USA), excised, cut into 2 × 2 mm cubes, and digested overnight at 37 °C with 12.5 ng/μl sequencing grade trypsin (Promega, Madison, WI, USA) at a ratio of 5:1 protein/trypsin (w/w) in 50 mM ammonium bicarbonate, pH 8.8, containing 10% (v/v) acetonitrile [35]. The resulting tryptic peptides from each band were extracted by incubation for 30 min in 12 mM ammonium bicarbonate, pH 8.8. Trifluoroacetic acid was added to a final concentration of 0.1%, and the peptides were finally desalted onto OMIX C18 pipette tips (Agilent Technologies, Santa Clara, CA, USA), dried down, and stored at −20 °C until use for mass spectrometry analysis. The desalted protein digests were resuspended in 0.1% formic acid and analyzed by reversed-phase liquid chromatography coupled to mass spectrometry (RP-LC–MS/MS) using an EASY-nLC II system coupled online to an LTQ Linear Ion Trap mass spectrometer (Thermo Fisher Scientific). The peptides were concentrated using a 0.1× 20 mm C18 reversed-phase (RP) precolumn (Thermo Fisher Scientific) and separated using a 0.075× 100 mm C18 RP column (Thermo Fisher Scientific) operating at 0.3 μl/min. Peptides were eluted using a 60-min gradient from 5 to 40% solvent B in solvent A (solvent A: 0.1% formic acid in water, solvent B: 0.1% formic acid, 80% acetonitrile in water). Electrospray ionization (ESI) was carried out using a nano-bore emitter stainless steel ID 30 μm (Thermo Fisher Scientific) interface. Peptides were detected in survey scans from 400 to 1600 atomic mass units (amu, 1 μscan), followed by 15 data-dependent MS/MS scans (Top 15), using an isolation width of two mass-to-charge ratio units, normalized collision energy of 35%, and dynamic exclusion applied for periods of 30 s. Peptide identification from the MS/MS raw data was carried out using the SEQUEST algorithm (Proteome Discoverer 1.4; Thermo Fisher Scientific). A search was performed against the Ixodidae UniProt protein database (184,796 entries in July 2020). The following constraints were used for the searches: tryptic cleavage after Arg and Lys, up to two missed cleavage sites, and tolerance of 1 Da for precursor ions and 0.8 Da for MS/MS fragment ions, and the search was performed allowing optional methionine oxidation and cysteine carbamidomethylation. A search was performed against a decoy database in an integrated decoy approach. A false discovery rate (FDR) < 0.01 was considered as a condition for successful peptide assignments, and at least two peptides per protein was the condition for successful protein identification. Protein bands from the second gel were excised and cut into small cubes, covered with PBS with 0.1% SDS, and incubated in a rotator overnight at 4 °C. The supernatants containing the protein fractions were methanol/chloroform-precipitated, resuspended in PBS for quantification by bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, CA, USA), and stored at −20 °C until fish treatment.
Tick protein annotations
Tick proteins identified after proteomics analysis were annotated for Gene Ontology in UniProt (https://www.uniprot.org) and VectorBase (https://vectorbase.org/vectorbase/app/). Biological processes include annotations in Drosophila or human proteins that may be related to AGS when information is not available in tick species. Sequences from all identified proteins were used for Basic Local Alignment Search Tool (BLAST) analysis (UniProtKB reference proteomes plus Swiss-Prot; E-threshold = 10) in UniProt (Table 1, Additional file 1: Dataset S1). Additionally, for the secreted protein B7P208—salivary antigen p23 A0A0K8RKR7 (Table 1, Additional file 1: Dataset S1), match to 3UV1_A Chain(A) PDB structure of allergen from dust mite (https://www.rcsb.org/structure/3UV1) was predicted using PredictProtein (https://predictprotein.org) tool (identity = 0.20, expected value = 1e−28, matched length = 205 of 222 to A0A0K8RKR7) (Table 2, Additional file 1: Dataset S1).
Quantitation of tick saliva proteins and α-Gal content
Protein and α-Gal content in tick saliva were determined in whole saliva and protein, non-protein, and deglycosylated fractions (Fig. 2A). The α-Gal levels were determined by an in-house enzyme-linked immunosorbent assay (ELISA) using tick saliva fractions in comparison with pig kidney (α-Gal-positive control) and human promyelocytic leukemia HL60 cells ATCC CCL-240 (α-Gal-negative control) (Fig. 2B) as described previously [22]. Tick saliva was diluted 1:1 in PBS and used to quantify α-Gal and protein content. Tick saliva, pig kidney, and HL60 protein concentrations were determined using a BCA Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s recommendations. Briefly, ELISA plates were coated with 100 ng proteins per well from different samples in carbonate/bicarbonate buffer (Sigma-Aldrich), incubated overnight at 4 °C following five washes with PBS containing 0.05% Tween 20 (PBST), and unspecific unions blocked with 1% human serum albumin (HSA; Sigma-Aldrich). Anti-α-Gal epitope monoclonal antibodies (M86; Enzo Life Sciences Inc., Farmingdale, NY, USA) were added at 1:100 dilution in PBS and incubated for 1 h at 37 °C followed by four washes with PBST, and anti-mouse IgM (μ-chain-specific)-peroxidase antibodies produced in goat (Sigma-Aldrich) were added at 1:2000 dilution in PBS. The average value of the blanks (wells without sample proteins; n = 5) was subtracted from all reads, and the analysis was conducted using a calibration curve with 0.0 to 10.0 ng α-Gal (Galα1-3Gal-BSA, 3 atom spacer, product code NGP0203; Dextra, Shinfield, UK) and optical density (OD) values at 450 nm using Microsoft Excel for Mac (v. 16.26) to convert ELISA reader values to α-Gal content per sample (R2 = 0.96). Values for α-Gal content on each sample were represented as nanograms of α-Gal per microgram of proteins. As a control, wells coated with tick saliva protein fraction (n = 3) were incubated with secondary anti-mouse IgM-peroxidase antibodies alone, and α-Gal content values were below 0.0005 ng/µg proteins, thus ruling out non-specific reactions.
Wild-type adult (6–8-month-old) AB male and female zebrafish were provided by Dr. Juan Galcerán Sáez from the Instituto de Neurociencias (IN-CSIC-UMH, Sant Joan d’Alacant, Alicante, Spain) and certified by Biosait Europe S.L. (Barcelona, Spain; https://biosait.com) as free of major fish pathogens [24]. Zebrafish were maintained in a flow-through water system at 27 °C with a light/dark cycle of 14 h/10 h and were fed twice daily at 9:30 and 13:30 with dry fish feed (Premium food tropical fish, DAPC, Valladolid, Spain; 50–70 μg/fish). On day 2 and until the end of the experiment at day 8, fish were fed dog food (Classic Red, ACANA, Champion Petfoods LP, Edmonton, Canada; 150–200 μg/fish). The composition of fish feed (cereals, fish and fish byproducts, soya, yeast, crustaceans, and algae) and dog food (23% lamb meat meal, 22% steel-cut oats, 5% fresh ranch-raised beef, 5% fresh Yorkshire pork, 5% lamb fat, 4% raw grass-fed lamb, 2% whole oats, 2% fresh beef liver, 2% pork meat meal, 2% herring oil, 2% fresh pork liver, 1% fresh beef tripe, 0.1% freeze-dried beef liver, whole red lentils, whole green peas, whole green lentils, whole garbanzo beans, whole yellow peas, sun-cured alfalfa, lentil fiber, dried brown kelp, fresh pumpkin, fresh butternut squash, fresh parsnips, fresh green kale, fresh spinach, fresh carrots, fresh Red Delicious apples, fresh Bartlett pears, fresh cranberries, fresh blueberries, chicory root, turmeric root, milk thistle, burdock root, lavender, marshmallow root, and rosehips) were as used in previous zebrafish studies [24]. Zebrafish were euthanized by overdose of tricaine methane sulfonate (MS222, 200–300 mg/l) by prolonged immersion (https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=32ed904477ecfcc4b0ac4f7ece7483d888149694) [36].
Characterization of anti-tick protein IgM antibody titers in zebrafish
Tick salivary gland protein extracts were prepared from salivary glands of I. ricinus eight female ticks-pool feeding for 5 days. Salivary glands were resuspended in 100 µl 1% Triton X-100-PBS solution and vortexed three times for 30 s. Then, the suspension was digested through a pellet pestle (DWK Life Sciences Kontes™ Pellet Pestle) and sonicated three times for 3 min. The BCA protein assay (Bio-Rad) was used for total protein quantification. For ELISA IgM titers quantification, high absorption capacity polystyrene microtiter plates were coated with 50 ng per well of tick saliva proteins in carbonate/bicarbonate buffer (Sigma-Aldrich). After overnight incubation at 4 °C, coated plates were washed once with 200 µl PBST (Sigma-Aldrich) and then blocked with 100 µl per well of 5% skim milk (Condalab, Madrid, Spain) in PBST (blocking solution) at room temperature (RT) with gentle shaking. Zebrafish serum samples from different groups of treatment were added at 1:100 dilution in blocking solution and incubated at 37 °C for 1 h. Plates were washed three times with PBST and 100 µl per well of specific rabbit anti-zebrafish IgM antibody diluted at 1:1000 in blocking solution. Plates were then incubated for 1 h at RT with gentle shaking. Plates were washed three times with PBST. A goat anti-rabbit IgG-peroxidase conjugate (Sigma-Aldrich) was added at 1:1000 and incubated for 1 h at RT with agitation. After three washes with 100 µl per well of PBST, 100 µl/well of TMB One Solution (Promega) was added and incubated for 15 min at RT in the dark. Finally, the reaction was stopped with 50 µl/well of 2 N H2SO4 and the OD at 450 nm was measured in a spectrophotometer (Multiskan, Thermo Fisher Scientific).
Characterization of anti-α-Gal IgM antibody titers in zebrafish
The ELISA was conducted as for tick proteins, but plates were coated with 100 ng α-Gal (Galα1-3Gal-BSA, 3 atom spacer, approximately 1.82 × 1020 Gal epitopes/g [37]; product code NGP0203; Dextra, Shinfield, UK) per well in carbonate/bicarbonate buffer (Sigma-Aldrich), incubated overnight at 4 °C following five washes with PBST. Unspecific unions were blocked with 1% HSA (Sigma-Aldrich) for 1 h at RT. Serum peritoneal fluid samples were diluted (1:100, v/v) in blocking solution, followed by the addition of 100 μl/well and incubation for 1.5 h at 37 °C. Plates were washed three times with PBST, and 100 μl/well of rabbit anti-zebrafish IgM antibodies diluted (1:1,000, v/v) in blocking solution was added and incubated for 1 h at RT. Plates were washed with PBST, and goat anti-rabbit IgG-peroxidase conjugate (Sigma-Aldrich) diluted 1:3000 in blocking solution was added and incubated for 1 h at RT. After washes with PBST, 100 μl/well of TMB (Promega) was added and incubated for 15 min at RT. Reactions were stopped with 50 μl/well of 2N H2SO4, and the OD at 450 nm was measured in a spectrophotometer (Multiskan, Thermo Fisher Scientific). Only hemorrhagic type allergic reactions were associated with individual fishes treated with tick saliva, and thus a correlation analysis was conducted between anti-α-Gal IgM antibody titers and these signs in this group (P < 0.05; n = 6).
Characterization of anti-glycan IgM antibody response in zebrafish
The glycochip array containing 378 glycans (20 µM) and 225 bacterial polysaccharides (2 µg/ml) was prepared as previously described (Semiotik LLC, Russia) [38]. Pooled sera obtained from a previous experiment [39] of 10 zebrafish for each group immunized by immersion with bovine serum albumin (BSA) coated with α-Gal (α-Gal; Dextra, Shinfield, UK) and PBS-treated control were diluted 1:10 in PBST (Sigma-Aldrich) and incubated with glycochip arrays overnight at 4 °C in a humidified chamber. After thorough washing with PBST to remove the proteins, glycochips were incubated with IgGs from rabbits immunized with zebrafish IgM diluted 1:1000 in PBST for 45 min at 20 °C. Then, glycochips were washed with PBST and incubated with goat anti-rabbit IgG (H + L)-Alexa Fluor 532 nm (Thermo Fisher Scientific) diluted 1:1000 in PBST at 20 °C for 1 h. Fluorescence signal intensity corresponding to the antibodies bound to printed glycans was measured with a GenePix 4100A fluorescence scanner (Molecular Devices, San Jose, CA, USA) at 500 PMT and a resolution of 10 µm. The images were processed using ScanArray Express 4.0 (fixed circle method) and then by Microsoft Excel software. Six spots represent each oligosaccharide or polysaccharide on the array, and data are reported as median relative fluorescence units (RFU) of replicates, given as a percentage ratio of maximum RFU on the chip (normRFU). The normRFU above 10% was considered significant (Additional file 2: Dataset S2).
Statistical analyses
The incidence of allergic reactions, abnormal behavior and feeding patterns, and mortality in zebrafish were compared between treatments by one-way analysis of variance (ANOVA) test with post hoc Tukey honestly significant difference (HSD) test (P < 0.05; https://astatsa.com/OneWay_Anova_with_TukeyHSD/). Anti-tick proteins and anti-α-Gal IgM antibody titers (OD at 450 nm) in zebrafish were compared between treatments by one-way ANOVA test with Bonferroni–Holm multiple comparisons with only pairs relative to PBS simultaneously compared (P < 0.05; n = 6–10 biological replicates; https://astatsa.com/OneWay_Anova_with_TukeyHSD/).